Insights+: The US FDA New Drug Approvals in June 2023
Shots:
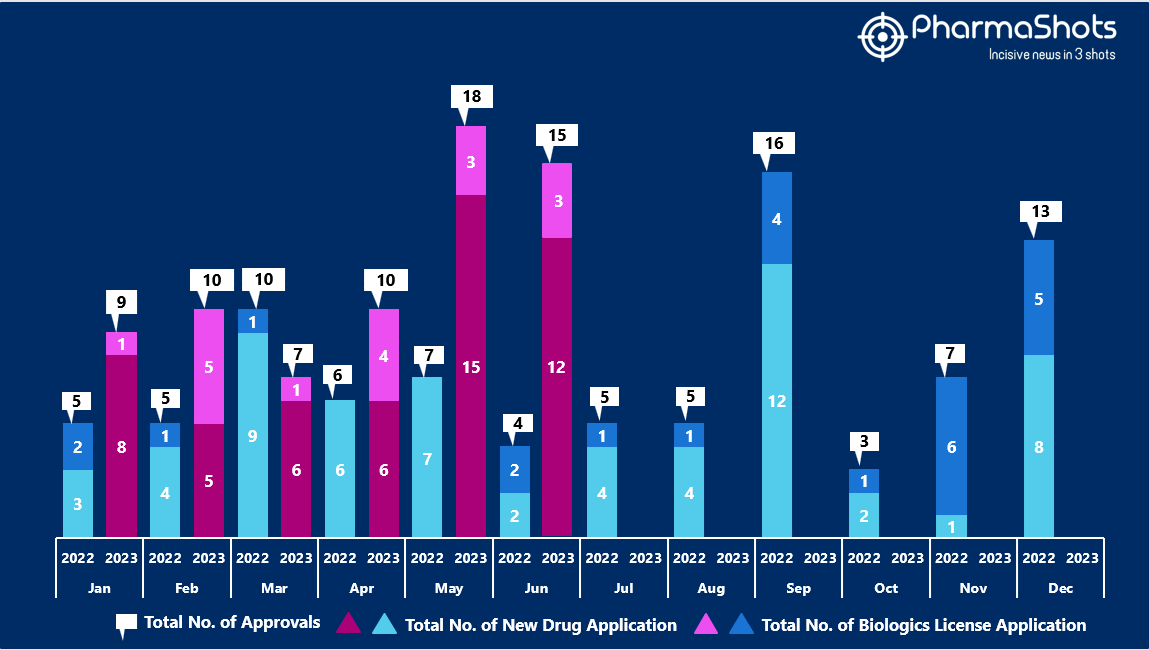
- The US FDA approved 12 NDAs and 3 BLA in June 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 69 novel products in 2023
- In June 2023, the major highlights drugs were Talzenna + Xtandi approval for prostate cancer, Rystiggo for generalized myasthenia gravis
- PharmaShots has compiled a list of a total of 15 new drugs approved by the US FDA in June 2023
Lynparza
Active ingredient: olaparib Approved: June 01, 2023
Company: AstraZeneca and MSD Disease: Prostate Cancer
- The US FDA has approved Lynparza + abiraterone & prednisone or prednisolone for deleterious or suspected deleterious BRCA-mutated mCRPC
- The approval was based on the subgroup analysis of the P-III trial (PROpel) evaluating Lynparza vs PBO in combination with abiraterone, prednisone, or prednisolone in 796 patients which showed an improvement in rPFS & OS over abiraterone alone, m-rPFS (not reached vs 8mos.) & m-OS (not reached vs 23mos.), 76% reduction in risk of disease progression or death, safety & tolerability profile was consistent with prior trials & known profiles of the individual therapies
- The combination therapy was approved in the EU & multiple other countries for mCRPC. Lynparza was approved in the US as monotx. for HRR gene-mutated mCRPC; and in the EU, Japan & China for BRCAm mCRPC
Prevymis
Active ingredient: letermovir Approved: June 09, 2023
Company: Merck Disease: Cytomegalovirus Disease
- The US FDA has approved Prevymis for prophylaxis of CMV disease in adult kidney transplant recipients who are at high risk (Donor CMV-seropositive/Recipient CMV-seronegative [D+/R-])
- The approval was based on the P-III trial of Prevymis vs valganciclovir in a ratio (1:1) in 601 patients which showed that Prevymis was non-inferior to valganciclovir for 1EPs of incidence of CMV disease @52wk. post-kidney transplant, patients with CMV disease (10% vs 12%), efficacy was comparable across all subgroups
- AEs leading to treatment discontinuation (4% vs 14%). Prevymis was approved in the US for prophylaxis of CMV inf. & in adults who are CMV-seropositive [R+] & received an allogeneic HSCT; approved in 60+ countries incl. EU member states, Canada, Japan & China
Linzess
Active ingredient: linaclotide Approved: June 13, 2023
Company: Ironwood Pharmaceuticals Disease: Functional Constipation
- The US FDA has approved Linzess (qd) for pediatric patients aged 6-17yrs. with functional constipation. Linzess is being developed & marketed by AbbVie & Ironwood in the US
- The approval was based on the P-III study evaluating Linzess (72mcg) vs PBO in a ratio (1:1) in 328 patients aged 6-17yrs. which showed a clinical improvement over PBO in 12wk. spontaneous bowel movement (SBM) frequency rate (SBMs/week)
- The results also showed a greater than two-fold least squares mean change from baseline in SBMs/week (2.6 vs 1.3). Ironwood also collaborated with AstraZeneca to develop & commercialize Linzess in China & with AbbVie for the development & commercialization of linaclotide in all other territories globally
Bylvay
Active ingredient: odevixibat Approved: June 14, 2023
Company: Ipsen Disease: Cholestatic Pruritus
- The US FDA has approved Bylvay, a non-systemic ileal bile acid transport inhibitor for patients with cholestatic pruritus aged 12mos. with ALGS
- The approval was based on the P-III study (ASSERT) evaluating odevixibat (120µg/kg/day) for 24wks. in patients aged 0-17yrs. The study met 1EPs & showed an improvement in pruritus, ≥90% were pruritus responders (≥1 point change at any time during 24wks.), the overall incidence of TEAEs was similar to PBO & no patients discontinued the study, 96% rolled over into the OLE study
- Bylvay was approved in the US for pruritus in patients aged ≥3mos. with PFIC & also approved in the EU for PFIC in patients aged ≥6mos. Bylvay is immediately available via prescription for eligible ALGS patients
Columvi
Active ingredient: glofitamab-gxbm Approved: June 16, 2023
Company: Genentech Disease: Diffuse Large B-cell Lymphoma
- The US FDA has approved Columvi, a CD20xCD3 T-cell engaging bispecific Ab to treat adult patients with r/r DLBCL. The therapy will be commercially available in the US in the coming weeks
- The approval was based on the P-I/II dose-escalation and expansion study (NP30179) evaluating Columvi in 860 patients showed that the patients treated with fixed-duration Columvi achieved durable remission, ORR (56%), CR (43%), patients continued to respond for 9mos. (68.5%), m-DoR (1.5yrs.). The study results were published in the NEJM
- Cytokine release syndrome (70%), 52% experienced grade 1, and 14% grade 2. The company plans to provide patient assistance programs through Genentech Access Solutions
Apoquel
Active ingredient: oclacitinib Approved: June 19, 2023
Company: Zoetis Disease: Pruritus
- The US FDA has approved Apoquel Chewable (oclacitinib chewable tablet) for the control of pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs aged ≥1yr.
- Zoetis continues to lead in dermatology innovation with a new formulation and shows after the first dosage comparable efficacy to that of Apoquel (oclacitinib tablet)
- In the US field test, 120 pet dogs received 1662 doses of Apoquel Chewable & an authorized dose range of 0.4-0.6mg/kg was given BID for ~14 days with the first 7 days of treatment used to assess palatability. The study discovered that a total of 1522 doses of Apoquel Chewable (91.6%) were freely accepted within 5min.
Talzenna
Active ingredient: talazoparib Approved: June 20, 2023
Company: Pfizer Disease: Prostate Cancer
- The US FDA has approved Talzenna + Xtandi for adult patients with HRR gene-mutated mCRPC. The approval was based on the P-III trial (TALAPRO-2) incl. 2 patient cohorts i.e., cohort 1 (all-comers; n=805) & cohort 2 (those with HRR (mutations; n=399) evaluating Talzenna + Xtandi vs PBO + Xtandi in 1125 patients
- The results showed a 55% reduction in risk of disease progression or death with prospectively identified HRR gene mutations (ATM, ATR, BRCA1, BRCA2, CDK12, CHEK2, FANCA, MLH1, MRE11A, NBN, PALB2, or RAD51C). The safety was consistent with the known safety profile of each therapy
- Serious ARs (30%) & discontinuation of Talzenna (10%). The results from the (TALAPRO-2) cohort 1 were published in The Lancet while the final OS data is expected in 2024
Lodoco
Active ingredient: colchicine Approved: June 20, 2023
Company: Agepha Pharma Disease: Cardiovascular Disease
- The US FDA has approved Lodoco for adult patients with atherosclerotic disease or with multiple risk factors for cardiovascular disease. Lodoco is expected to be available in H2’23
- The approval was based on the clinical trial evaluating Lodoco in 5522 patients with chronic coronary disease. The results showed that Lodoco (0.5mg) was found to significantly reduce the overall risk of CV death, spontaneous MI, ischemic stroke, or ischemia-driven coronary revascularization by 31% over PBO when added to high-intensity statins & other cardiology prevention therapies
- Lodoco, is the first anti-inflammatory atheroprotective cardiovascular treatment that can be used alone or in combination with cholesterol-lowering medications
Vyvgart Hytrulo
Active ingredient: efgartigimod alfa and hyaluronidase-qvfc Approved: June 21, 2023
Company: argenx Disease: Generalized Myasthenia Gravis
- Halozyme reported that argenx received the US FDA’s approval of Vyvgart Hytrulo with Enhanze as SC use for gMG in adult patients who are AChR+. The product is expected to be available in the US in July 2023
- The approval was based on the P-III study (ADAPT-SC) evaluating Vyvgart Hytrulo vs Vyvgart (IV). The 1EPs of noninferiority were met & showed a mean total IgG reduction from baseline @29 Day (66.4% vs 62.2%) & the key 2EPs were met which were consistent with efficacy measures from the (ADAPT) study. The results were consistent with the (ADAPT) clinical trial
- The new SC formulation can be administered as a single inj. (1008mg fixed dose) by a healthcare professional. The MAA for efgartigimod (SC) is under EMA & PMDA review with an expected decision at the end of 2023 & Q1’24, respectively
Jardiance and Synjardy
Active ingredient: empagliflozin Approved: June 21, 2023
Company: Boehringer Ingelheim Disease: Type 2 Diabetes
- The US FDA has approved Jardiance (empagliflozin) and Synjardy (empagliflozin and metformin hydrochloride) as additions to diet and exercise to improve blood sugar control in children aged ≥10yrs. with T2D
- The P-III trial evaluating empagliflozin vs PBO in 157 patients aged 10-17yrs. with inadequately controlled T2D. The results showed that patients treated with empagliflozin were superior in reducing hemoglobin A1c @26wks.
- 52 patients had an average 0.2% decrease in hemoglobin A1c over 0.7% increase in hemoglobin A1c in the 53 patients taking PBO, reductions in fasting plasma glucose after not eating or drinking for 8hrs. Jardiance & Synjardy was approved in the US in 2014 & 2015, respectively.
Litfulo
Active ingredient: ritlecitinib Approved: June 26, 2023
Company: Pfizer Disease: Alopecia Areata
- The approval was based on the P-IIb/III trial (ALLEGRO) evaluating Litfulo vs PBO in 718 patients aged 12yrs. with ≥50% scalp hair loss as measured SALT at 118 sites in 18 countries. Patients initially assigned to PBO switched to Litfulo (50 or 200mg loading dose + 50mg) for an additional 24wks.
- The results showed that 23% vs 1.6% treated with Litfulo (50mg) had ≥80% scalp hair coverage (SALT≤20) after 6mos. The efficacy & safety of Litfulo were consistent b/w adolescents (12-17yrs.) & adults (≥18yrs.), AEs reported in 4% & the results were published in The Lancet
- The EMA has accepted the MAA for ritlecitinib with an expected decision in Q3’23. Litfulo will be available in the coming weeks & is being studied for vitiligo, CD & UC
Rystiggo
Active ingredient: rozanolixizumab Approved: June 27, 2023
Company: UCB Disease: Generalized Myasthenia Gravis
- The US FDA has approved Rystiggo for the treatment of adult patients with gMG who are AchR or anti-muscle-specific tyrosine kinase (MuSK) Ab+. The approval was based on the P-III study (MycarinG) evaluating rozanolixizumab in 200 adult patients with gMG with an open-label extension
- The results showed that patients treated with rozanolixizumab resulted in significant improvements in gMG-specific outcomes, incl. everyday activities i.e., breathing, talking, swallowing & being able to rise from a chair, a significant difference was seen in MG-ADL & QMG total score change from baseline
- The product is expected to be commercially available in the US in Q3’23. Rozanolixizumab is currently under EMA & Japan review for the treatment of adults with gMG
13. Pfizer’s Ngenla Receives the US FDA’s Approval for Pediatric Growth Hormone Deficiency
Ngenla
Active ingredient: somatrogon Approved: June 28, 2023
Company: Pfizer Disease: Pediatric Growth Hormone Deficiency
- The US FDA has approved Ngenla for the treatment of pediatric patients aged ≥3yrs. who have growth failure due to inadequate secretion of endogenous growth hormone. The therapy is expected to be available in the US in Aug 2023
- The approval was based on the P-III trial results evaluating the safety and efficacy of Ngenla (qw) vs somatropin (qd). The study met its 1EPs which showed that Ngenla was found to be non-inferior over somatropin as measured by annual height velocity at 12mos., was generally well tolerated & had a safety profile comparable to somatropin
- Ngenla was approved for the treatment of pediatric GHD in 40+ markets incl. Canada, Australia, Japan & EU member states
14. CellTrans’ Lantidra Receives the US FDA’s Approval for the Treatment of Type 1 Diabetes
Lantidra
Active ingredient: donislecel Approved: June 29, 2023
Company: CellTrans Disease: Type 1 Diabetes
- The approval was granted based on the study evaluating the safety & effectiveness of Lantidra in patients (n=30) with type 1 diabetes who are unable to approach target glycated hemoglobin due to current repeated episodes of severe hypoglycemia
- The results from the study depicted that out of the evaluable patients, 21 did not require insulin for ≥1yr.,11 for 1-5yrs. & 10 for ≥5yrs. As per the safety data, adverse reactions depicted in patients were associated with the number of infusions & follow-up time
- Lantidra is an allogeneic pancreatic islet cellular therapy developed from deceased donor pancreatic cells that treat type 1 diabetes through infused allogeneic islet beta cells
15. BioMarin’s Roctavian Receives the US FDA’s Approval for the Treatment of Severe Hemophilia A
Roctavian
Active ingredient: valoctocogene roxaparvovec Approved: June 30, 2023
Company: BioMarin Disease: Severe Hemophilia A
- The approval was based on the P-III (GENEr8-1) study evaluating Roctavian in patients (n=134) with severe hemophilia A. For 112 patients, baseline ABR data were collected during 6mos. on FVIII prophylaxis before receiving Roctavian & for 22 patients, baseline ABR was collected retrospectively
- The results showed that in 112 patients, a mean ABR reduction of 52% was reported after receiving Roctavian by the end of follow-up (2.6 bleeds/year) vs baseline ABR while receiving routine FVIII prophylaxis (5.4 bleeds/year) & rate of spontaneous bleeds & joint bleeds was also reduced with Roctavian (0.5 & 0.6 vs 2.3 &3.1 bleeds/year)
- BioMarin expects to continue to monitor the long-term effects of the treatment with a follow-up of 15yrs.
Related Post: Insights+: The US FDA New Drug Approvals in May 2023
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.